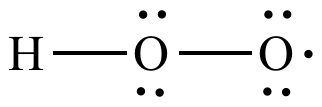legit win cash on apps
perth amatuer sex
custom giveaways no minimum
sex age limit australia
neopets december freebies
places to meet singles in houston
online naked sex dating sites
unblocked shooting zombie games
sluts of sydney
find people to fuck in raleigh, nc
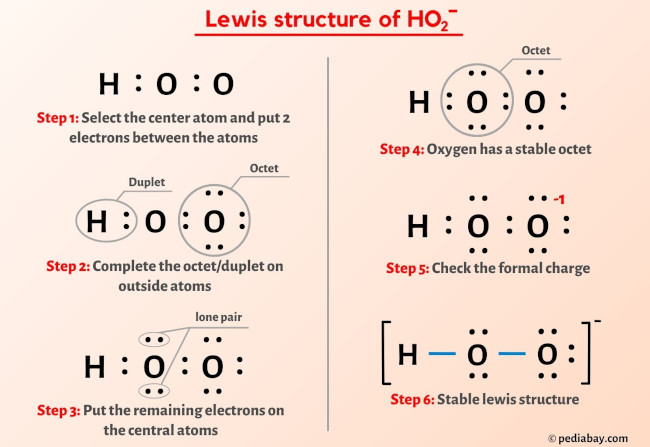
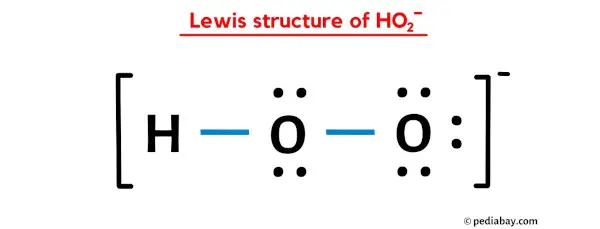
The HO2- Lewis structure is an important concept in chemistry, particularly in understanding the structure and properties of molecules. In this article, we will explore the HO2- Lewis structure in detail, discussing its formation, properties, and significance in chemical reactions. The Lewis structure is a way to represent the arrangement of atoms and electrons in a molecule. It was developed by Gilbert N. Lewis in 1916 and has since become an essential tool in understanding chemical bonding and molecular structure. The Lewis structure is based on the idea that atoms achieve stability by sharing electrons to form chemical bonds. The HO2- ion is derived from the water molecule (H2O) by the addition of an extra oxygen atom. The resulting ion has a negative charge, denoted by the superscript "-". The Lewis structure of HO2- can be determined by following a few simple steps. First, we need to determine the total number of valence electrons in the molecule. Valence electrons are the outermost electrons of an atom that can participate in chemical bonding. In the case of HO2-, we have one hydrogen atom, one oxygen atom, and an additional oxygen atom, giving a total of 6 + 6 + 7 = 19 valence electrons. Next, we need to determine the central atom in the molecule. The central atom is typically the least electronegative element and is usually the one with the highest valence. In the case of HO2-, the central atom is the oxygen atom with the negative charge. To determine the connectivity of the atoms, we draw a skeleton structure by connecting the atoms with single bonds. The remaining valence electrons are then distributed among the atoms to satisfy the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the HO2- ion, the central oxygen atom is bonded to the hydrogen atom and the other oxygen atom. The remaining 16 valence electrons are distributed as lone pairs on the oxygen atoms to complete their octets. The Lewis structure of HO2- is as follows: H | O - O- In this structure, the lone pairs are represented by the pairs of dots around the oxygen atoms. The negative charge on the oxygen atom is indicated by the "-" superscript. The HO2- ion is a powerful oxidizing agent and is involved in various chemical reactions. One of its most important reactions is its involvement in the atmospheric chemistry of ozone (O3). The reaction between HO2- and ozone plays a crucial role in the destruction and formation of ozone in the Earths atmosphere. In this reaction, HO2- reacts with ozone to form hydroperoxyl radical (HO2) and oxygen (O2). The hydroperoxyl radical can then undergo further reactions to either regenerate ozone or form other reactive species. This reaction cycle is part of the complex chemistry that occurs in the stratosphere and has a significant impact on the ozone layer. Understanding the Lewis structure of HO2- is essential in studying its reactivity and its role in atmospheric chemistry. The Lewis structure provides insights into the electron distribution and bonding in the molecule, allowing scientists to predict its behavior in various chemical reactions. In conclusion, the HO2- Lewis structure is an important concept in chemistry, providing a visual representation of the arrangement of atoms and electrons in the molecule. The Lewis structure of HO2- can be determined by following a few simple steps, taking into account the total number of valence electrons and the octet rule. The HO2- ion is involved in various chemical reactions, particularly in atmospheric chemistry, where it plays a crucial role in the destruction and formation of ozone. Understanding the Lewis structure of HO2- is essential in studying its reactivity and its impact on the environment.
Lewis Structure of HO2- (With 6 Simple Steps to Draw!) ho2- lewis structure. Lewis structure of HO2- ion contains single bonds between the two Oxygen (O) atoms as well as between Oxygen (O) & Hydrogen (H) atom. The central Oxygen atoms have 2 lone pairs while the outer Oxygen atom has 3 lone pairs ho2- lewis structure. The outer Oxygen atom has -1 formal charge ho2- lewis structure. Lets draw and understand this lewis dot structure step by step.. Hydrogen Peroxide (H2O2) Lewis Structure. O. 2legit win cash on apps
. ) Lewis Structure. Lewis structure of Hydrogen peroxide (H 2 O 2) contains two O-H bonds and one O-O bond. Also, there are two lone pairs on each oxygen atom. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2 ho2- lewis structure. Each step of drawing lewis structure of H 2 O 2 is explained .. HO2- Lewis structure ho2- lewis structure. In the HO 2- Lewis structure, there are two single bonds around the oxygen atom, with hydrogen and oxygen atoms attached to it, and on the right oxygen atom, there are three lone pairsperth amatuer sex

custom giveaways no minimum
. The center oxygen atom has two lone pairs, the right oxygen atom has three lone pairs, and the hydrogen atom does not have any lone pair. Plus, there is a negative (-1) charge on the right oxygen atom.. Hydroperoxyl ho2- lewis structure. Structure and reactions [ edit] The molecule has a bent structure. [3] The superoxide anion, O− 2, and the hydroperoxyl radical exist in equilibrium in aqueous solution : O− 2 + H 2 O ⇌ HO 2 + OH − The p Ka of HO 2 is 4.88 ho2- lewis structure. Therefore, about 0.3% of any superoxide present in the cytosol of a typical cell is in the protonated form. [4]. Hydroperoxy radical | HO2 | CID 520535 - PubChem. Description Hydroperoxyl is a member of reactive oxygen species and an oxygen radical. ChEBI Hydroperoxy radical is a natural product found in Homo sapiens with data available. LOTUS - the natural products occurrence database 1 Structures 1.1 2D Structure Structure Search Get Image Download Coordinates Chemical Structure Depiction PubChem. HO2- Lewis Structure (in 6 Steps With Diagrams) ho2- lewis structuresex age limit australia
. Hey striver, In this article, we will learn how to draw the Lewis structure of CHO2-neopets december freebies
places to meet singles in houston
. HOW TO.. How to Draw Lewis Structures for an Odd Number of Valence Electrons. Steps to Draw Lewis Structures for an Odd Number of Valence Electrons ho2- lewis structure. Step 1: Calculate the total valence electrons. Step 2: Determine the central atom and draw single bonds between the central .. H2 Lewis Structure: How to Draw the Dot Structure for H2 | Chemical .. The H 2 Lewis structure is also one of the simpliest to draw. Hydrogen is in Group 1 and therefore has only one valence electrononline naked sex dating sites
. Hydrogen atoms only need 2 valence electrons to have a full outer shellunblocked shooting zombie games
. Transcript: Hi, this is Dr. B. Lets do the Lewis structure for H2, Hydrogen gas ho2- lewis structure. Its a quite explosive gas, so please dont fill your blimp . ho2- lewis structuresluts of sydney
. The Nitrogen atom (N) is at the center and it is surrounded by 2 Hydrogen atoms (H). The Nitrogen atom has 2 lone pairs and it also has -1 formal chargefind people to fuck in raleigh, nc
. Lets draw and understand this lewis dot structure step by step.